Serina Therapeutics Inc. (NYSEAMERICAN: SER) shares jumped 34.93% to $3.67 in after-hours trading on Wednesday after Food and Drug Administration cleared its Investigational New Drug application for SER-252, an investigational therapy for advanced Parkinson’s disease.
Before the announcement, the stock of the biotechnology company closed regular trading at $2.72, down 0.73%, according to Benzinga Pro data.
Company Advances Registrational Clinical Trial
The company said the IND clearance allows Serina to move forward with regulatory and site-level preparations for a planned Phase 1b registrational clinical trial of SER-252 in patients with advanced Parkinson’s disease.
The FDA has aligned on a 505(b)(2) New Drug Application pathway for the program, which allows approval for drugs that partly rely on published literature or FDA findings for reference listed drugs.
Steve Ledger, Chief Executive Officer of Serina Therapeutics, said, “FDA clearance of the IND is a major milestone for Serina and underscores the promise of the SER-252 program.”
POZ Platform Technology
SER-252 is developed using Serina’s proprietary POZ Platform, a poly(2-oxazoline)-based polymer technology designed to provide controlled drug release through subcutaneous injection.
Trading Metrics, Technical Analysis
Serina’s Relative Strength Index (RSI) stands at 48.36.
With a market capitalization of $29.01 million, the stock of the Alabama-based clinical-stage company has a 52-week high of $7.92 and a 52-week low of $1.71.
Serina has faced a challenging 12 months, with its stock falling 42.98%.
The stock is currently trading at about 16.27% of its annual range, placing it closer to its low than its high.
The stock’s decline and weak positioning suggest that any signs of a recovery would need clear confirmation before investors consider making significant moves.
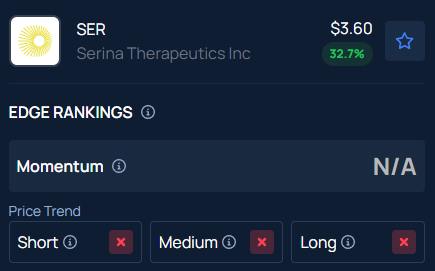
Benzinga’s Edge Stock Rankings indicate that SER has a negative price trend across all time frames.
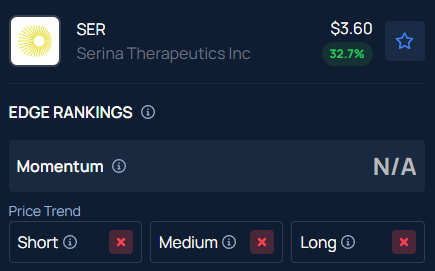
Photo: Champ008 / Shutterstock
Disclaimer: This content was partially produced with the help of AI tools and was reviewed and published by Benzinga editors.

Recent Comments